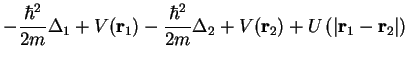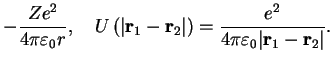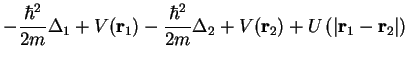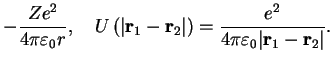Next: Perturbation theory in
Up: Introduction into Many-Particle Systems
Previous: Direct and Exchange Term:
Contents
Index
Here, we deal with the Helium atom (
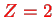 ) , Lithium ion Li
) , Lithium ion Li
 (
(
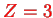 ), Beryllium ion Be
), Beryllium ion Be
 (
(
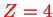 ) etc.
These are two-electron systems as in section III.2.1 with Hamiltonian Eq. (III.2.17),
) etc.
These are two-electron systems as in section III.2.1 with Hamiltonian Eq. (III.2.17),
Subsections
Tobias Brandes
2005-04-26