




Next: The Rayleigh-Ritz Variational Method
Up: The Hydrogen Molecule Ion
Previous: The Hydrogen Molecule Ion
Contents
Index
(Cf. Weissbluth [4] ch. 26 for this section). The Hamiltonian for the electronic part at fixed positions
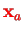 and
and
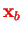 of the two protons is a Hamiltonian for a single electron at position
of the two protons is a Hamiltonian for a single electron at position
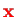 ,
,
![$\displaystyle \mathcal{H}^{(0)}_{\rm e}= \frac{{\bf p}^2}{2m}-\frac{e^2}{4\pi\v...
...f x}-{\bf x}_a\vert}+\frac{1}{\vert{\bf x}-{\bf x}_b\vert} -\frac{1}{R}\right],$](img821.png) |
|
|
(3.1) |
where
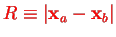 and the (fixed) Coulomb repulsion energy
and the (fixed) Coulomb repulsion energy
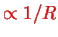 between the two nuclei has been included for later convenience.
The eigenstates of this Hamiltonian can be determined from an exact solution in ellipsoidal coordinates. The corresponding wave functions are called molecular orbitals (MO) because these orbitals spread out over the whole molecule.
between the two nuclei has been included for later convenience.
The eigenstates of this Hamiltonian can be determined from an exact solution in ellipsoidal coordinates. The corresponding wave functions are called molecular orbitals (MO) because these orbitals spread out over the whole molecule.
Instead of discussing the exact solution, it is more instructive to discuss an approximate method that can also be used for more complicated molecules. This method is called LCAO (linear combination of atomic orbitals) and has a centrol role in quantum chemistry.





Next: The Rayleigh-Ritz Variational Method
Up: The Hydrogen Molecule Ion
Previous: The Hydrogen Molecule Ion
Contents
Index
Tobias Brandes
2005-04-26