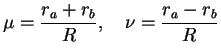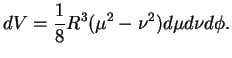Next: Symmetries of MOs in
Up: Bonding and Antibonding
Previous: Rayleigh-Ritz Results
Contents
Index
This is required in order to find the values for the two energies
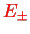 , and also in order to find out which of the two states
, and also in order to find out which of the two states
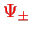 has lower energy!
The calculations are performed by introducing elliptical coordinates
has lower energy!
The calculations are performed by introducing elliptical coordinates
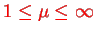 ,
,
 ,
,
 ,
,
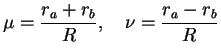 |
|
|
(3.20) |
and noting that the volume element in these coordinates is
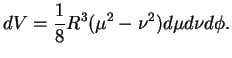 |
|
|
(3.21) |
The result for
 ,
,
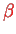 , and
, and
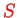 is found as a function of the (fixed) distance
is found as a function of the (fixed) distance
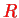 between the two protons. Using this together with Eq. (V.3.13), one finally obtains
between the two protons. Using this together with Eq. (V.3.13), one finally obtains
Be careful because I haven't checked these explicit expressions, which are from Atkins/Friedman [5] ch. 8.3.
Figure:
Energy level splitting for 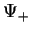 (a) and
(a) and 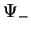 (b), from Weissbluth [4].
(b), from Weissbluth [4].
|
|
REMARKS:
The charge distribution in
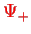 and
and
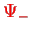 is shown in Fig.(V.3.3.2).
is shown in Fig.(V.3.3.2).
Figure:
Charge distribution in 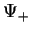 (a) and
(a) and 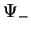 (b), from Weissbluth [4].
(b), from Weissbluth [4].
|
|





Next: Symmetries of MOs in
Up: Bonding and Antibonding
Previous: Rayleigh-Ritz Results
Contents
Index
Tobias Brandes
2005-04-26