




Next: Ground State Energy
Up: Hartree-Fock Equations
Previous: Exchange Term
Contents
Index
In the case
 , we are back to the Helium atom (
, we are back to the Helium atom (
 electrons). We assume
electrons). We assume
 |
|
|
(3.32) |
i.e. we only have two spin orbitals with opposite spin. From the Hartree-Fock equations Eq. (E.4.2), we obtain
Formally, these are still two equations due to the label
 (
(
 ), but the two equations are the same and we may set
), but the two equations are the same and we may set
 . The sum in the exchange part has only one term which is half the direct part, and therefore (we re-insert the explicit expression for
. The sum in the exchange part has only one term which is half the direct part, and therefore (we re-insert the explicit expression for
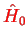 )
)
![$\displaystyle \left[ -\frac{\hbar^2}{2m}\Delta + V({\bf r}) + \int d{\bf r'} \v...
...})\vert^2 U(\vert{r-r'}\vert) \right]
\psi({\bf r})= \varepsilon \psi({\bf r}).$](img726.png) |
|
|
(3.34) |
Since we have only one orbital wave function, we only have one equation.





Next: Ground State Energy
Up: Hartree-Fock Equations
Previous: Exchange Term
Contents
Index
Tobias Brandes
2005-04-26
![$\displaystyle \left[ \hat{H_0} + {\sum_{i=1}^2 \int d{\bf r'} \vert\psi({\bf r'})\vert^2 U(\vert{r-r'}\vert)} \right]
\psi({\bf r})$](img721.png)

![$\displaystyle \left[ -\frac{\hbar^2}{2m}\Delta + V({\bf r}) + \int d{\bf r'} \v...
...})\vert^2 U(\vert{r-r'}\vert) \right]
\psi({\bf r})= \varepsilon \psi({\bf r}).$](img726.png)