




Next: Ground state
Up: Two-electron Atoms and Ions
Previous: Two-electron Atoms and Ions
Contents
Index
A problem with perturbation theory here is the fact that the interaction
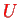 between the two electrons is not small.
between the two electrons is not small.
The unperturbed states
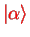 and
and
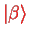 for the orbital wave functions (cf. section III.2.3.2) are the eigenstates of the hydrogen problem, Eq. (II.1.20),
for the orbital wave functions (cf. section III.2.3.2) are the eigenstates of the hydrogen problem, Eq. (II.1.20),
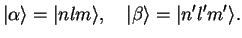 |
|
|
(3.2) |
Note that these do not contain a spin index.
Subsections
Tobias Brandes
2005-04-26
![]() and
and
![]() for the orbital wave functions (cf. section III.2.3.2) are the eigenstates of the hydrogen problem, Eq. (II.1.20),
for the orbital wave functions (cf. section III.2.3.2) are the eigenstates of the hydrogen problem, Eq. (II.1.20),